6. Oncogenes
Marco A. Pierotti, PhD
Gabriella Sozzi, PhD
Carlo M. Croce, MD
Since the early proposals of Boveri more than a century ago, much
experimental evidence has confirmed that, at the molecular level, cancer is a
result of lesions in the cellular deoxyribonucleic acid (DN)A.
First, it has been observed that a cancer cell transmits to its daughter cells
the phenotypic features characterizing the cancerous state. Second, most of the
recognized mutagenic compounds are also carcinogenic, having as a target
cellular DNA. Finally, the karyotyping of several types of human tumors,
particularly those belonging to the hematopoietic system, led to the
identification of recurrent qualitative and numerical chromosomal aberrations,
reflecting pathologic re-arrangements of the cellular genome. Taken together,
these observations suggest that the molecular pathogenesis of human cancer is
due to structural and/or functional alterations of specific genes whose normal
function is to control cellular growth and differentiation or, in different
terms, cell birth and cell death.1,2
The identification and characterization of the genetic elements playing a
role in the scenario of human cancer pathogenesis have been made possible by
the development of DNA recombinant techniques during the last two decades. One
milestone was the use of the DNA transfection technique that helped clarify the
cellular origin of the “viral oncogenes.” The latter were previously
characterized as the specific genetic elements capable of conferring the
tumorigenic properties to the ribonucleic acid (RNA) tumor viruses also known
as retroviruses.3,4 Furthermore, the transfection technique led to the
identification of cellular transforming genes that do not have a viral
counterpart. Besides the source of their original identification, viral or
cellular genome, these transforming genetic elements have been designated as
protooncogenes in their normal physiologic version and oncogenes when altered
in cancer.5,6 A second relevant experimental approach
has regarded the identification and characterization of clonal and recurrent
cytogenetic abnormalities in cancer cells, especially those derived from the
hematopoietic system. Several oncogenes have been thus defined by molecular
cloning of the chromosomal breakpoints, including translocations and
inversions. Additional oncogenes have been identified through the analysis of
chromosomal regions anomalously stained (homogeneously staining regions),
representing gene amplification. Finally, the detection of chromosome deletions
has been instrumental in the process of identification and cloning of a second
class of cancer-associated genes, the tumor suppressors. Contrary to the
oncogenes that are activated by dominant mutations and whose activity is to
promote cell growth, tumor suppressors act in the normal cell as negative
controllers of cell growth and are inactive in tumor cells. In general,
therefore, the mutations inactivating tumor suppressor genes are of the
recessive type.7
Recently, a third class of cancer-associated genes has been defined
thanks to the analysis of tumors of a particular type; that is, tumors in which
an inherited mutated predisposing gene plays a significant role. These tumors
include cancers in patients suffering from hereditary nonpolyposis colorectal
cancer syndromes.
The genes implicated in these tumors have been defined as mutator genes
or genes involved in the DNA-mismatch repair process. Although not directly
involved in the carcinogenesis process, these genes, when inactivated, expose
the cells to a very high mutagenic load that eventually may involve the
activation of oncogenes and the inactivation of tumor suppressors.8
In this chapter, the methods by which oncogenes were discovered will be
first described. The various functions of cellular protooncogenes will then be
presented, and the genetic mechanisms of protooncogene activation will be
summarized. Finally, the role of specific oncogenes in the initiation and
progression of human tumors will be discussed.
Discovery and identification
of oncogenes
The first oncogenes were discovered through the study of retroviruses,
RNA tumor viruses whose genomes are reverse-transcribed into DNA in infected
animal cells.9 During the course of infection,
retroviral DNA is inserted into the chromosomes of host cells. The integrated
retroviral DNA, called the provirus, replicates along with the cellular DNA of
the host.10 Transcription of the DNA provirus leads to the production of viral
progeny that bud through the host cell membrane to infect other cells. Two
categories of retroviruses are classified by their time course of tumor
formation in experimental animals. Acutely transforming retroviruses can
rapidly cause tumors within days after injection. These retroviruses can also
transform cell cultures to the neoplastic phenotype. Chronic or weakly
oncogenic retroviruses can cause tissue-specific tumors in susceptible strains
of experimental animals after a latency period of many months. Although weakly
oncogenic retroviruses can replicate in vitro, these viruses do not transform
cells in culture.
Retroviral oncogenes are altered versions of host cellular protooncogenes
that have been incorporated into the retroviral genome by recombination with
host DNA, a process known as retroviral transduction.11 This surprising
discovery was made through study of the Rous sarcoma virus (RSV) (Figure 6-1).
RSV is an acutely transforming retrovirus first isolated from a chicken sarcoma
over 80 years ago by Peyton Rous.12 Studies of RSV mutants in the early 1970s
revealed that the transforming gene of RSV was not required for viral
replication.13–15 Molecular hybridization studies then showed that the RSV
transforming gene (designated v-src) was homologous to a host cellular gene
(c-src) that was widely conserved in eukaryotic species.16 Studies of many
other acutely transforming retroviruses from fowl, rodent, feline, and nonhuman
primate species have led to the discovery of dozens of different retroviral
oncogenes (see below and Table 6-1). In every case, these retroviral oncogenes
are derived from normal cellular genes captured from the genome of the host.
Viral oncogenes are responsible for the rapid tumor formation and efficient in
vitro transformation activity characteristic of acutely transforming retroviruses.
In contrast to acutely transforming retroviruses, weakly oncogenic
retroviruses do not carry viral oncogenes. These retroviruses, which include
mouse mammary tumor virus (MMTV) and various animal leukemia viruses, induce
tumors by a process called insertional mutagenesis (Figure 6-2).8 This process results from integration of the DNA provirus
into the host genome in infected cells. In rare cells, the provirus inserts
near a protooncogene. Expression of the protooncogene is then abnormally driven
by the transcriptional regulatory elements contained within the long terminal
repeats of the provirus.17,18 In these cases, proviral
integration represents a mutagenic event that activates a protooncogene.
Activation of the protooncogene then results in transformation of the cell,
which can grow clonally into a tumor. The long latent period of tumor formation
of weakly oncogenic retroviruses is therefore due to the rarity of the provirus
insertional event that leads to tumor development from a single transformed cell.
Insertional mutagenesis by weakly oncogenic retroviruses, first demonstrated in
bursal lymphomas of chickens, frequently involves the same oncogenes (such as
myc, myb, and erb B) that are carried by acutely transforming
retroviruses.19–21 In many cases, however, insertional mutagenesis has been
used as a tool to identify new oncogenes, including int-1, int-2, pim-1, and
lck.22
The demonstration of activated protooncogenes in human tumors was first
shown by the DNA-mediated transformation technique.23,24 This technique, also
called gene transfer or transfection assay, verifies the ability of donor DNA
from a tumor to transform a recipient strain of rodent cells called NIH 3T3, an
immortalized mouse cell line (Figure 6-3).25,26 This sensitive assay, which can
detect the presence of single-copy oncogenes in a tumor sample, also enables
the isolation of the transforming oncogene by molecular cloning techniques.
After serial growth of the transformed NIH 3T3 cells, the human tumor oncogene
can be cloned by its association with human repetitive DNA sequences. The first
human oncogene isolated by the gene transfer technique was derived from a
bladder carcinoma.27,28 Overall, approximately 20% of
individual human tumors have been shown to induce transformation of NIH 3T3
cells in gene-transfer assays. The value of transfection assay was recently
reinforced by the laboratory of Robert Weinberg, which showed that the ectopic
expression of the telomerase catalytic subunit (hTERT), in combination with the
simian virus 40 large T product and a mutated oncogenic H-ras protein, resulted
in the direct tumorigenic conversion of normal human epithelial and fibroblast
cells.29 Many of the oncogenes identified by gene-transfer studies are
identical or closely related to those oncogenes transduced by retroviruses.
Most prominent among these are members of the ras family that have been
repeatedly isolated from various human tumors by gene transfer.30,31 A number
of new oncogenes (such as neu, met, and trk) have also been identified by the
gene-transfer technique.32,33 In many cases, however, oncogenes identified by
gene transfer were shown to be activated by rearrangement during the
experimental procedure and are not activated in the human tumors that served as
the source of the donor DNA, as in the case of ret that was subsequently found
genuinely rearranged and activated in papillary thyroid carcinomas.34–36
Chromosomal translocations have served as guideposts for the discovery of
many new oncogenes.37,38 Consistently recurring
karyotypic abnormalities are found in many hematologic and solid tumors. These
abnormalities include chromosomal rearrangements as well as the gain or loss of
whole chromosomes or chromosome segments. The first consistent karyotypic
abnormality identified in a human neoplasm was a characteristic small
chromosome in the cells of patients with chronic myelogenous leukemia.39 Later
identified as a derivative of chromosome 22, this abnormality was designated
the
Oncogenes, protooncogenes,
and their functions
Protooncogenes encode proteins that are involved in the control of cell
growth. Alteration of the structure and/or expression of protooncogenes can
activate them to become oncogenes capable of inducing in susceptible cells the
neoplastic phenotype. Oncogenes can be classified into five groups based on the
functional and biochemical properties of protein products of their normal
counterparts (proto-oncogenes). These groups are (1) growth factors, (2) growth
factor receptors, (3) signal transducers, (4) transcription factors, and (5)
others, including programmed cell death regulators. Table 6-1 lists examples of
oncogenes according to their functional categories.
Growth Factors
Growth factors are secreted polypeptides that function as extracellular
signals to stimulate the proliferation of target cells.41,42
Appropriate target cells must possess a specific receptor in order to respond
to a specific type of growth factor. A well-characterized example is
platelet-derived growth factor (PDGF), an approximately 30 kDa protein
consisting of two polypeptide chains.43 PDGF is released from
platelets during the process of blood coagulation. PDGF stimulates the
proliferation of fibroblasts, a cell growth process that plays an important
role in wound healing. Other well-characterized examples of growth factors
include nerve growth factor, epidermal growth factor, and fibroblast growth
factor.
The link between growth factors and retroviral oncogenes was revealed by
study of the sis oncogene of simian sarcoma virus, a retrovirus first isolated
from a monkey fibrosarcoma. Sequence analysis showed that sis encodes the beta
chain of PDGF.44 This discovery established the
principle that inappropriately expressed growth factors could function as
oncogenes. Experiments demonstrated that the constitutive expression of the sis
gene product (PDGF-β)
was sufficient to cause neoplastic transformation of fibroblasts but not of
cells that lacked the receptor for PDGF.45 Thus, transformation by sis requires
interaction of the sis gene product with the PDGF receptor. The mechanism by
which a growth factor affects the same cell that produces it is called
autocrine stimulation .46 The constitutive expression
of the sis gene product appears to cause neoplastic transformation by the
mechanism of autocrine stimulation, resulting in self-sustained aberrant cell
proliferation. This model, derived from experimental animal systems, has been
recently demonstrated in a human tumor. Dermatofibrosarcoma protuberans (DP) is
an infiltrative skin tumor that was demonstrated to present specific
cytogenetic features: reciprocal translocation and supernumerary ring
chromosomes, involving chromosomes 17 and 22.47,48 Molecular cloning of the
breakpoints revealed a fusion between the collagen type Ia1 (COL1A1) gene and
PDGF-β gene. The fusion gene
resulted in a deletion of PDGF-β exon 1 and a constitutive release of this growth factor.49 Subsequent
experiments of gene transfer of DPs genomic DNA into NIH 3T3 cells directly
demonstrated the occurrence of an autocrine mechanism by the human rearranged
PDGF-b gene involving the activation of the endogenous PDGF receptor.50,51 Another example of a growth factor that can function as
an oncogene is int-2, a member of the fibroblast growth factor family. Int-2 is
sometimes activated in mouse mammary carcinomas by MMTV insertional
mutagenesis.52top link
Growth Factor Receptors
Some viral oncogenes are altered versions of normal growth factor
receptors that possess intrinsic tyrosine kinase activity.53 Receptor tyrosine
kinases, as these growth factor receptors are collectively known,
have a characteristic protein structure consisting of three principal domains:
(1) the extracellular ligand-binding domain, (2) the transmembrane domain, and
(3) the intracellular tyrosine kinase catalytic domain (see Figure 6-2). Growth
factor receptors are molecular machines that transmit information in a
unidirectional fashion across the cell membrane. The binding of a growth factor
to the extracellular ligandbinding domain of the receptor results in the
activation of the intracellular tyrosine kinase catalytic domain. The
recruitment and phosphorylation of specific cytoplasmic proteins by the
activated receptor then trigger a series of biochemical events generally
leading to cell division.
Because of the role of growth factor receptors in the regulation of
normal cell growth, it is not surprising that these receptors constitute an
important class of protooncogenes. Examples include erb B, erb B-2, fms, kit,
met, ret, ros, and trk. Mutation or abnormal expression of growth factor
receptors can convert them into oncogenes.54 For example, deletion of the
ligand-binding domain of erb B (the epidermal growth factor receptor) is
thought to result in constitutive activation of the receptor in the absence of
ligand binding.55 Point mutation in the tyrosine kinase domain or of the
extracellular domain and deletion of intracellular regulatory domains can also
result in the constitutive activation of receptor tyrosine kinases. Increased
expression through gene amplification and abnormal expression in the wrong cell
type are additional mechanisms through which growth factor receptors may be
involved in neoplasia. The identification and study of altered growth factor
receptors in experimental models of neoplasia have contributed much to our
understanding of the normal regulation of cell proliferation.
Signal Transducers
Mitogenic signals are transmitted from growth factor receptors on the
cell surface to the cell nucleus through a series of complex interlocking
pathways collectively referred to as the signal transduction cascade.56 This relay of information is accomplished in part by the
stepwise phosphorylation of interacting proteins in the cytosol. Signal
transduction also involves guanine nucleotide-binding proteins and second
messengers such as the adenylate cyclase system.57 The
first retroviral oncogene discovered, src, was subsequently shown to be
involved in signal transduction.
Many protooncogenes are members of signal transduction pathways.58,59 These consist of two main groups: nonreceptor protein
kinases and guanosine triphosphate (GTP)-binding proteins. The nonreceptor
protein kinases are subclassified into tyrosine kinases (eg, abl, lck, and src)
and serine/threonine kinases (eg, raf-1, mos, and pim-1). GTP-binding proteins
with intrinsic GTPase activity are subdivided into monomeric and heterotrimeric
groups.60 Monomeric GTP-binding proteins are members of the important ras
family of protooncogenes that includes H-ras, K-ras, and N-ras.61
Heterotrimeric GTP-binding proteins (G proteins) implicated as protooncogenes
currently include gsp and gip. Signal transducers are often converted to
oncogenes by mutations that lead to their unregulated activity, which in turn
leads to uncontrolled cellular proliferation.62
Transcription Factors
Transcription factors are nuclear proteins that regulate the expression
of target genes or gene families.63 Transcriptional regulation is mediated by
protein binding to specific DNA sequences or DNA structural motifs, usually located
upstream of the target gene. Transcription factors often belong to multigene
families that share common DNA-binding domains such as zinc fingers. The
mechanism of action of transcription factors also involves binding to other
proteins, sometimes in heterodimeric complexes with specific partners.
Transcription factors are the final link in the signal transduction pathway
that converts extracellular signals into modulated changes in gene expression.
Many protooncogenes are transcription factors that were discovered
through their retroviral homologs.64 Examples include erb A, ets, fos, jun,
myb, and c-myc. Together, fos and jun form the AP-1 transcription factor, which
positively regulates a number of target genes whose expression leads to cell
division.65,66 Erb A is the receptor for the T3 thyroid hormone,
triiodothyronine.67 Protooncogenes that function as transcription factors are
often activated by chromosomal translocations in hematologic and solid
neoplasms.68 In certain types of sarcomas, chromosomal translocations cause the
formation of fusion proteins involving the association of EWS gene with various
partners and resulting in an aberrant tumor-associated transcriptional
activity. Interestingly, a role of the adenovirus E1A gene in promoting the
formation of fusion transcript fli1/ews in normal human fibroblasts was
recently reported.69 An important example of a protooncogene with a
transcriptional activity in human hematologic tumors is the c-myc gene, which
helps to control the expression of genes leading to cell proliferation.70 As
will be discussed later in this chapter, the cmyc gene is frequently activated
by chromosomal translocations in human leukemia and lymphoma.
Programmed Cell Death
Regulation
Normal tissues exhibit a regulated balance between cell proliferation and
cell death. Programmed cell death is an important component in the processes of
normal embryogenesis and organ development. A distinctive type of programmed
cell death, called apoptosis, has been described for mature tissues.71 This process is characterized morphologically by blebbing of
the plasma membrane, volume contraction, condensation of the cell nucleus, and
cleavage of genomic DNA by endogenous nucleases into nucleosome-sized
fragments. Apoptosis can be triggered in mature cells by external stimuli such
as steroids and radiation exposure. Studies of cancer cells have shown that
both uncontrolled cell proliferation and failure to undergo programmed cell
death can contribute to neoplasia and insensitivity to anticancer treatments.
The only protooncogene thus far shown to regulate programmed cell death
is bcl-2. Bcl-2 was discovered by the study of chromosomal translocations in
human lymphoma.72,73 Experimental studies show that bcl-2 activation inhibits
programmed cell death in lymphoid cell populations.74 The dominant mode of
action of activated bcl-2 classifies it as an oncogene. The bcl-2 gene encodes
a protein localized to the inner mitochondrial membrane, endoplasmic reticulum,
and nuclear membrane. The mechanism of action of the bcl-2 protein has not been
fully elucidated, but studies indicate that it functions in part as an
antioxidant that inhibits lipid peroxidation of cell membranes.75 The normal function of bcl-2 requires interaction with other
proteins, such as bax, also thought to be involved in the regulation of
programmed cell death (Figure 6-4). It is unlikely that bcl-2 is the only
apoptosis gene involved in neoplasia although additional protooncogenes await
identification.
Mechanisms of oncogene
activation
The activation of oncogenes involves genetic changes to cellular
protooncogenes. The consequence of these genetic alterations is to confer a
growth advantage to the cell. Three genetic mechanisms activate oncogenes in
human neoplasms: (1) mutation, (2) gene amplification, and (3) chromosome
rearrangements. These mechanisms result in either an alteration of
protooncogene structure or an increase in protooncogene expression (Figure
6-5). Because neoplasia is a multistep process, more than one of these mechanisms
often contribute to the genesis of human tumors by
altering a number of cancer-associated genes. Full expression of the neoplastic
phenotype, including the capacity for metastasis, usually involves a
combination of protooncogene activation and tumor suppressor gene loss or
inactivation.
Mutation
Mutations activate protooncogenes through structural alterations in their
encoded proteins. These alterations, which usually involve critical protein
regulatory regions, often lead to the uncontrolled, continuous activity of the
mutated protein. Various types of mutations, such as base substitutions,
deletions, and insertions, are capable of activating protooncogenes.76 Retroviral oncogenes, for example, often have deletions that
contribute to their activation. Examples include deletions in the aminoterminal
ligand-binding domains of the erb B, kit, ros, met, and trk oncogenes.6 In human tumors, however, most characterized oncogene
mutations are base substitutions (point mutations) that change a single amino
acid within the protein.
Point mutations are frequently detected in the ras family of
protooncogenes (K-ras, H-ras, and N-ras).77 It has been estimated that as many
as 15% to 20% of unselected human tumors may contain a ras mutation. Mutations
in K-ras predominate in carcinomas. Studies have found K-ras mutations in about
30% of lung adenocarcinomas, 50% of colon carcinomas, and 90% of carcinomas of
the pancreas.78 N-ras mutations are preferentially found in hematologic
malignancies, with up to a 25% incidence in acute myeloid leukemias and
myelodysplastic syndromes.79,80 The majority of thyroid carcinomas have been
found to have ras mutations distributed among K-ras, H-ras, and N-ras, without
preference for a single ras family member but showing an association with the
follicular type of differentiated thyroid carcinomas.81,82 The majority of ras
mutations involve codon 12 of the gene, with a smaller number involving other
regions such as codons 13 or 61.83 Ras mutations in human tumors have been
linked to carcinogen exposure. The consequence of ras mutations is the
constitutive activation of the signal-transducing function of the ras protein.
Another significant example of activating point mutations is represented
by those affecting the ret protooncogene in multiple endocrine neoplasia type
2A syndrome (MEN2A).
Germline point mutations affecting one of the cysteines located in the
juxtamembrane domain of the ret receptor have been found to confer an oncogenic
potential to the latter as a consequence of the ligand-independent activation
of the tyrosine kinase activity of the receptor. Experimental evidences have
pointed out that these mutations involving cysteine residues promote ret
homodimerization via the formation of intermolecular disulfide bonding, most
likely as a result of an unpaired number of cysteine residues.84,85
Gene Amplification
Gene amplification refers to the expansion in copy number of a gene
within the genome of a cell. Gene amplification was first discovered as a
mechanism by which some tumor cell lines can acquire resistance to
growth-inhibiting drugs.86 The process of gene amplification occurs through
redundant replication of genomic DNA, often giving rise to karyotypic
abnormalities called double-minute chromosomes (DMs) and homogeneous staining
regions (HSRs).87 DMs are characteristic minichromosome structures without
centromeres. HSRs are segments of chromosomes that lack the normal alternating
pattern of light- and dark-staining bands. Both DMs and HSRs represent large
regions of amplified genomic DNA containing up to several hundred copies of a
gene. Amplification leads to the increased expression of genes, which in turn
can confer a selective advantage for cell growth.
The frequent observation of DMs and HSRs in human tumors suggested that
the amplification of specific protooncogenes may be a common occurrence in
neoplasia.88 Studies then demonstrated that three protooncogene families-myc,
erb B, and ras-are amplified in a significant number of human tumors (Table
6-2). About 20% to 30% of breast and ovarian cancers show c-myc amplification,
and an approximately equal frequency of c-myc amplification is found in some
types of squamous cell carcinomas.89 N-myc was discovered as a new member of
the myc protooncogene family through its amplification in neuroblastomas.90
Amplification of N-myc correlates strongly with advanced tumor stage in
neuroblastoma (Table 6-3), suggesting a role for this gene in tumor
progression.91,92 L-myc was discovered through its amplification in small-cell
carcinoma of the lung, a neuroendocrine-derived tumor.93 Amplification of erb
B, the epidermal growth factor receptor, is found in up to 50% of glioblastomas
and in 10% to 20% of squamous carcinomas of the head and neck.77 Approximately
15% to 30% of breast and ovarian cancers have amplification of the erbB-2
(HER-2/neu) gene. In breast cancer, erbB-2 amplification correlates with
advanced stage and poor prognosis.94 Members of the ras gene family, including
K-ras and N-ras, are sporadically amplified in various carcinomas.
Chromosomal Rearrangements
Recurring chromosomal rearrangements are often detected in hematologic
malignancies as well as in some solid tumors.37,95,96 These rearrangements
consist mainly of chromosomal translocations and, less frequently, chromosomal
inversions. Chromosomal rearrangements can lead to hematologic malignancy via
two different mechanisms: (1) the transcriptional activation of protooncogenes
or (2) the creation of fusion genes. Transcriptional activation, sometimes
referred to as gene activation, results from chromosomal rearrangements that
move a proto-oncogene close to an immunoglobulin or T-cell receptor gene (see
Figure 6-5). Transcription of the protooncogene then falls under control of
regulatory elements from the immunoglobulin or T-cell receptor locus. This
circumstance causes deregulation of protooncogene expression, which can then
lead to neoplastic transformation of the cell.
Fusion genes can be created by chromosomal rearrangements when the
chromosomal breakpoints fall within the loci of two different genes. The
resultant juxtaposition of segments from two different genes gives rise to a
composite structure consisting of the head of one gene and the tail of another.
Fusion genes encode chimeric proteins with transforming activity. In general,
both genes involved in the fusion contribute to the transforming potential of
the chimeric oncoprotein. Mistakes in the physiologic rearrangement of
immunoglobulin or T-cell receptor genes are thought to give rise to many of the
recurring chromosomal rearrangements found in hematologic malignancy.97
Examples of molecularly characterized chromosomal rearrangements in hematologic
and solid malignancies are given in Table 6-4. In some cases, the same
protooncogene is involved in several different translocations (ie, c-myc, ews,
and ret).
Gene Activation
The t(8;14)(q24;q32) translocation, found in
about 85% of cases of Burkitt lymphoma, is a well-characterized example of the
transcriptional activation of a proto-oncogene. This chromosomal rearrangement
places the c-myc gene, located at chromosome band 8q24, under control of
regulatory elements from the immunoglobulin heavy chain locus located at
14q32.98 The resulting transcriptional activation of c-myc, which encodes a
nuclear protein involved in the regulation of cell proliferation, plays a
critical role in the development of Burkitt lymphoma.99 The c-myc gene is also
activated in some cases of Burkitt lymphoma by translocations involving
immunoglobulin light-chain genes.100,101 These are t(2;8)(p12;q24), involving
the κ locus located at 2p12, and t(8;22)(q24;q11), involving the κ
locus at 22q11 (Figure 6-6). Although the position of the chromosomal
breakpoints relative to the c-myc gene may vary considerably in individual
cases of Burkitt lymphoma, the consequence of the translocations is the same:
deregulation of c-myc expression, leading to uncontrolled cellular
proliferation.
In some cases of T cell acute lymphoblastic leukemia (T-ALL), the c-myc
gene is activated by the t(8;14)(q24;q11)
translocation. In these cases, transcription of c-myc is placed under the
control of regulatory elements within the T-cell receptor α locus located
at 14q11.102 In addition to c-myc, several protooncogenes that encode nuclear
proteins are activated by various chromosomal translocations in T-ALL involving
the T-cell receptor α or β locus. These include HOX11, TAL1, TAL2,
and RBTN1/Tgt1.103–105 The proteins encoded by these
genes are thought to function as transcription factors through DNA-binding and
protein-protein interactions. Overexpression or inappropriate expression of
these proteins in T cells is thought to inhibit T-cell differentiation and lead
to uncontrolled cellular proliferation.
A number of other protooncogenes are also activated by chromosomal
translocations in leukemia and lymphoma. In most follicular lymphomas and some
large cell lymphomas, the bcl-2 gene (located at 18q21) is activated as a
consequence of t(14;18)(q32;q21) translocations.72,73 Overexpression of the
bcl-2 protein inhibits apoptosis, leading to an imbalance between lymphocyte
proliferation and programmed cell death.74 Mantle cell lymphomas are
characterized by the t(11;14)(q13;q32) translocation, which activates the
cyclin d1 (bcl-1) gene located at 11q13.106,107 Cyclin D1 is a G1 cyclin
involved in the normal regulation of the cell cycle. In some cases of T cell
chronic lymphocytic leukemia and prolymphocytic leukemia, the tcl-1 gene at
14q32.1 is activated by inversion or translocation involving chromosome 14.108 The tcl-1 gene product is a small cytoplasmic protein whose
function is not yet known.
Gene Fusion
The first example of gene fusion was discovered through the cloning of
the breakpoint of the Philadelphia chromosome in chronic myelogenous leukemia
(CML).109 The t(9;22)(q34;q11) translocation in CML fuses the c-abl gene,
normally located at 9q34, with the bcr gene at 22q11 (Figure 6-7).110 The
bcr/abl fusion, created on the der(22) chromosome, encodes a chimeric protein
of 210 kDa, with increased tyrosine kinase activity and abnormal cellular
localization.111 The precise mechanism by which the bcr/abl fusion protein
contributes to the expansion of the neoplastic myeloid clone is not yet known.
The t(9;22) translocation is also found in up to 20%
of cases of acute lymphoblastic leukemia (ALL). In these cases, the breakpoint
in the bcr gene differs somewhat from that found in CML, resulting in a 185 kDa
bcr/abl fusion protein.112 It is unclear at this time
why the slightly smaller bcr/abl fusion protein leads to such a large
difference in neoplastic phenotype.
In addition to c-abl, two other genes encoding tyrosine kinases are
involved in distinct gene fusion events in hematologic malignancy. The t(2;5)(p23;q35) translocation in anaplastic large cell
lymphomas fuses the NPM gene (5q35) with the ALK gene (2p23).113 ALK encodes a
membranespanning tyrosine kinase similar to members of the insulin growth
factor receptor family. The NPM protein is a nucleolar phosphoprotein involved
in ribosome assembly. The NPM/ALK fusion creates a chimeric oncoprotein in
which the ALK tyrosine kinase activity may be constitutively activated. The t(5;12)(q33;p13) translocation, characterized in a case of
chronic myelomonocytic leukemia, fuses the tel gene (12p13) with the tyrosine kinase
domain of the PDGF receptor b gene (PDGFR-b at 5q33).114 The tel gene is
thought to encode a nuclear DNA-binding protein similar to those of the ets
family of protooncogenes.
Gene fusions sometimes lead to the formation of chimeric transcription
factors.68,95 The t(1;19)(q23;p13) translocation,
found in childhood pre-B-cell ALL, fuses the E2A transcription factor gene
(19p13) with the PBX1 homeodomain gene (1q23).115 The E2A/PBX1 fusion protein
consists of the amino-terminal transactivation domain of the E2A protein and
the DNA-binding homeodomain of the PBX1 protein. The t(15;17)(q22;q21)
translocation in acute promyelocytic leukemia (PML) fuses the PML gene (15q22)
with the RARA gene at 17q21.116 The PML protein contains a zinc-binding domain
called a RING finger that may be involved in protein-protein interactions. RARA
encodes the retinoic acid alpha-receptor protein, a member of the nuclear
steroid/thyroid hormone receptor superfamily. Although retinoic acid binding is
retained in the fusion protein, the PML/RARA fusion protein may confer altered
DNA-binding specificity to the RARA ligand complex.117 Leukemia patients with
the PML/RARA gene fusion respond well to retinoid treatment. In these cases,
treatment with all-trans retinoic acid induces differentiation of PML cells.
The ALL1 gene, located at chromosome band 11q23, is involved in
approximately 5% to 10% of acute leukemia cases overall in children and
adults.118,119 These include cases of ALL, acute
myeloid leukemia, and leukemias of mixed cell lineage. Among leukemia genes,
ALL1 (also called MLL and HRX) is unique because it participates in fusions
with a large number of different partner genes on the various chromosomes. Over
20 different reciprocal translocations involving the ALL1 gene at 11q23 have
been reported, the most common of which are those involving chromosomes 4, 6,
9, and 19.120 In approximately 5% of cases of acute leukemia in adults, the
ALL1 gene is fused with a portion of itself.121 This special type of gene
fusion is called self-fusion.122 Self-fusion of the ALL1 gene, which is thought
to occur through a somatic recombination mechanism, is found in high incidence
in acute leukemias with trisomy 11 as a sole cytogenetic abnormality. The ALL1
gene encodes a large protein with DNA-binding motifs, a transactivation domain,
and a region with homology to the Drosophila trithorax protein (a regulator of
homeotic gene expression).123,124 The various partners
in ALL1 fusions encode a diverse group of proteins, some of which appear to be
nuclear proteins with DNA-binding motifs.125,126 The ALL1 fusion protein
consists of the aminoterminus of ALL1 and the carboxyl terminus of one of a
variety of fusion partners. It appears that the critical feature in all ALL1
fusions, including self-fusion, is the uncoupling of the ALL1 amino-terminal
domains from the remainder of the ALL1 protein.
Solid tumors, especially sarcomas, sometimes have consistent chromosomal
translocations that correlate with specific histologic types of tumors.127 In
general, translocations in solid tumors result in gene fusions that encode
chimeric oncoproteins. Studies thus far indicate that in sarcomas, the majority
of genes fused by translocations encode transcription factors.128 In myxoid
liposarcomas, the t(12;16)(q13;p11) fuses the FUS (TLS) gene at 16p11 with the
CHOP gene at 12q13.129 The FUS protein contains a transactivation domain that
is contributed to the FUS/CHOP fusion protein. The CHOP protein, which is a
dominant inhibitor of transcription, contributes a protein-binding domain and a
presumptive DNA-binding domain to the fusion. Despite knowledge of these
structural features, the mechanism of action of the FUS/CHOP oncoprotein is not
yet known. In
In DP, an infiltrating skin tumor, both a reciprocal translocation t(17;22)(q22;q13) and supernumerary ring chromosomes derived
from the t(17;22) have been described.
Although early successful studies in this field have been performed with
lymphomas and leukemia, as we have discussed before, the first chromosomal
abnormality in solid tumors to be characterized at the molecular level as a fusion
protein was an inversion of chromosome 10 found in papillary thyroid
carcinomas.132 In this tumor, two main recurrent structural changes have been
described, including inv(10) (q112.2; q21.2), as the
more frequent alteration, and a t(10;17)(q11.2;q23). These two abnormalities
represent the cytogenetic mechanisms which activate the protooncogene ret on
chromosome 10, forming the oncogenes RET/ptc1 and RET/ptc2, respectively.
Alterations of chromosome 1 in the same tumor type have then been associated to
the activation of NTRK1 (chromosome 1), an NGF receptor which, like RET, forms
chimeric fusion oncogenic proteins in papillary thyroid carcinomas.133 A
comparative analysis of the oncogenes originated from the activation of these
two tyrosine kinase receptors has allowed the identification and
characterization of common cytogenetic and molecular mechanisms of their
activation. In all cases, chromosomal rearrangements fuse the tK portion of the
two receptors to the 5′ end of different genes that, due to their general
effect, have been designated as activating genes. In the majority of cases, the
latter belong to the same chromosome where the related receptor is located, 10
for RET and 1 for NTRK1.
Furthermore, although functionally different, the various activating
genes share the following three properties: (1) they are ubiquitously
expressed; (2) they display domains demonstrated or predicted to be able to
form dimers or multimers; (3) they translocate the tK-receptor-associated
enzymatic activity from the membrane to the cytoplasm.
These characteristics can explain the mechanism(s) of oncogenic
activation of ret and NTRK1 protooncogenes. In fact, following the fusion of
their tK domain to activating gene, several things happen: (1) ret and NTRK1,
whose tissue-specific expression is restricted to subsets of neural cells,
become expressed in the epithelial thyroid cells; (2) their dimerization
triggers a constitutive, ligand-independent transautophosphorylation of the
cytoplasmic domains and as a consequence, the latter can recruit SH2 and SH3
containing cytoplasmic effector proteins, such as Shc and Grb2 or phospholipase
C (PLCγ), thus inducing a constitutive mitogenic pathway; (3) the
relocalization in the cytoplasm of ret and NTRK1 enzymatic activity could allow
their interaction with unusual substrates, perhaps modifying their functional
properties.
In conclusion, in PTCs, the oncogenic activation of ret and NTRK1
protooncogenes following chromosomal rearrangements occurring in breakpoint
cluster regions of both protooncogenes could be defined as an ectopic,
constitutive, and topologically abnormal expression of their associated
enzymatic (tK) activity.134top link
Oncogenes in the initiation
and progression of neoplasia
Human neoplasia is a complex multistep process involving sequential
alterations in protooncogenes (activation) and in tumor suppressor genes
(inactivation). Statistical analysis of the age incidence of human solid tumors
indicates that five or six independent mutational events may contribute to
tumor formation.135 In human leukemias, only three or
four mutational events may be necessary, presumably involving different genes.
The study of chemical carcinogenesis in animals provides a foundation for
our understanding the multistep nature of cancer.136 In
the mouse model of skin carcinogenesis, tumor formation involves three phases,
termed initiation, promotion, and progression. Initiation of skin tumors can be
induced by chemical mutagens such as 7,12-dimethyl-benzanthracene
(DMBA) (Figure 6-8). After application of DMBA, the mouse skin appears normal.
If the skin is then continuously treated with a promoter, such as the phorbol
ester TPA, precancerous papillomas will form. Chemical promoters such as TPA
stimulate growth but are not mutagenic substances. Over a period of months of
continuous application of the promoting agent, some of the papillomas will
progress to skin carcinomas. Treatment with DMBA or TPA alone does not cause
skin cancer. Mouse papillomas initiated with DMBA usually have H-ras oncogenes
with a specific mutation in codon 61 of the H-ras gene. The mouse skin tumor
model indicates that initiation of papillomas is the result of mutation of the
H-ras gene in individual skin cells by the chemical mutagen DMBA. For
papillomas to appear on the skin, however, growth of mutated cells must be
continuously stimulated by a promoting agent. Additional unidentified genetic
changes must then occur for papillomas to progress to carcinoma.
Although a single oncogene is sufficient to cause tumor formation by some
rapidly transforming retroviruses such as RSV, transformation by a single
oncogene is not usually seen in experimental models of cancer. Other rapidly
transforming retroviruses carry two different oncogenes that cooperate in
producing the neoplastic phenotype. One well-characterized example of this type
of cooperation is the avian erythroblastosis virus, which carries the erb A and
erb B oncogenes.137 Cooperation between oncogenes can also be demonstrated by
in vitro transformation studies using nonimmortalized cell lines. For example,
studies have shown cooperation between the nuclear myc protein and the
cytoplasmic-membrane-associated ras protein in the transformation of rat embryo
fibroblasts.138 As previously reported, a cooperation between SV40 large T
product and mutated H-ras gene also have been found necessary to transform
normal human epithelial and fibroblast cells provided that they constitutively
expressed the catalytic subunit of telomerase enzyme, indicating a more complex
pattern in the neoplastic conversion of human cells.
Collaboration between two different general categories of oncogenes (eg,
nuclear and cytoplasmic) can often be demonstrated but is not strictly required
for transformation.139 The production of transgenic mice expressing a single
oncogene such as myc has also demonstrated that multiple genetic changes are
necessary for tumor formation. These transgenic mice strains, in fact,
generally show an increased incidence of neoplasia and the tumors that result frequently
are clonal, implying that other events are necessary.The production of
transgenic mice expressing a single oncogene such as myc has also demonstrated
that multiple genetic changes are necessary for tumor formation.140
Cytogenetic studies of the clonal evolution of human hematologic
malignancies have provided much insight into the multiple steps involved in the
initiation and progression of human tumors.141 The
evolution of CML from chronic phase to acute leukemia is characterized by an
accumulation of genetic changes seen in the karyotypes of the evolving
malignant clones. The early chronic phase of CML is defined by the presence of
a single
The initiation and progression of human neoplasia involve the activation
of oncogenes and the inactivation or loss of tumor suppressor genes. The
mechanisms of oncogene activation and the time course of events, however, vary
among different types of tumors. In hematologic malignancies, soft-tissue
sarcomas and the papillary type of thyroid carcinomas, initiation of the
malignant process predominantly involves chromosomal rearrangements that
activate various oncogenes.95 Many of the chromosomal
rearrangements in leukemia and lymphoma are thought to result from errors in
the physiologic process of immunoglobulin or T-cell receptor gene rearrangement
during normal B-cell and T-cell development. Late events in the progression of
hematologic malignancies involve oncogene mutation, mainly of the ras family,
inactivation of tumor suppressor genes such as p53, and sometimes additional
chromosomal translocations.143
In carcinomas such as colon and lung cancer, the initiation of neoplasia
has been shown to involve oncogene and tumor suppressor gene mutations.144
These mutations are generally thought to result from chemical carcinogenesis,
especially in the case of tobacco-related lung cancer, where a novel tumor
suppressor gene (designated FHIT) has been found to be inactivated in the
majority of cancers, particularly in those from smokers.145,146 In
preneoplastic adenomas of the colon, the K-ras gene is often mutated.147
Progression of colon adenomas to invasive carcinoma frequently involves
inactivation or loss of the DCC and p53 tumor suppressor genes (see Figure
6-9). Gene amplification is often seen in the progression of some carcinomas
and other types of tumors. Amplification of the erb B-2 oncogene may be a late
event in the progression of breast cancer.94 Members of the myc oncogene family
are frequently amplified in small-cell carcinoma of the lung.93 As mentioned
previously, amplification of N-myc strongly correlates with the progression and
clinical stage of neuroblastoma.92 Although there is variability in the
pathways of human tumor initiation and progression, studies of various types of
malignancy have clearly confirmed the multistep nature of human cancer.
Summary and conclusions
The initiation and progression of human neoplasia is a multistep process
involving the accumulation of genetic changes in somatic cells. These genetic
changes consist of the activation of cooperating oncogenes and the inactivation
of tumor suppressor genes, which both appear necessary for a complete
neoplastic phenotype. Oncogenes are altered versions of normal cellular genes
called protooncogenes. Protooncogenes are a diverse group of genes involved in
the regulation of cell growth. The functions of protooncogenes include growth
factors, growth factor receptors, signal transducers, transcription factors,
and regulators of programmed cell death. Protooncogenes may be activated by
mutation, chromosomal rearrangement, or gene amplification. Chromosomal
rearrangements that include translocations and inversions can activate
proto-oncogenes by deregulation of their transcription (eg, transcriptional
activation) or by gene fusion. Tumor suppressor genes, which also participate
in the regulation of normal cell growth, are usually inactivated by point
mutations or truncation of their protein sequence coupled with the loss of the
normal allele.
The discovery of oncogenes represented a breakthrough in our
understanding of the molecular and genetic basis of cancer. Oncogenes have also
provided important knowledge concerning the regulation of normal cell
proliferation, differentiation, and programmed cell death. The identification
of oncogene abnormalities has provided tools for the molecular diagnosis and
monitoring of cancer. Most important, oncogenes represent potential targets for
new types of cancer therapies. It is more than a hope that a new generation of
chemotherapeutic agents directed at specific oncogene targets will be
developed. The goal of these new drugs will be to kill cancer cells selectively
while sparing normal cells. One promising approach entails using specific
oncogene targets to trigger programmed cell death. One example of the
accomplishment of such a goal is represented by the inhibition of the
tumor-specific tyrosine kinase bcr/abl in CML by imatinib (Gleevec or
STI571)148(see Figure 6-10). The same compound has been proven active also in a
different tumor type, gastrointestinal stromal tumor, where it inhibits the
tyrosine kinase receptor c-kit.149 Our rapidly
expanding knowledge of the molecular mechanisms of cancer holds great promise
for the development of better combined methods of cancer therapy in the near
future.
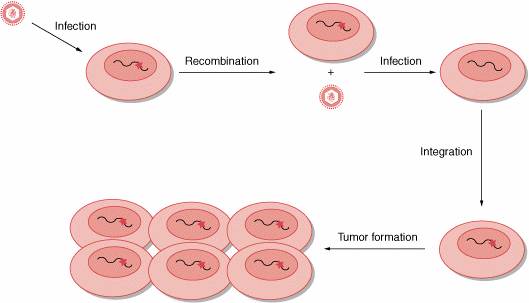
Figure 6-1. Retroviral transduction. A ribonucleic acid (RNA) tumor virus infects a
human cell carrying an activated src gene (red star). After the process
of recombination between retroviral genome and host deoxyribonucleic acid (DNA),
the oncogene c-src is incorporated into the retroviral genome and is
renamed v-src. When the retrovirus carrying v-src infects a human
cell, the viral oncogene is rapidly transcribed and is responsible for the
rapid tumor formation.
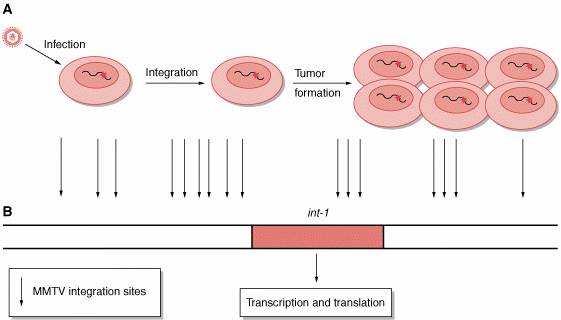
Figure 6-2. Insertional mutagenesis. A, The process is independent of genes carried by the retrovirus.
Retrovirus, for example, mouse mammary tumor virus (MMTV), infects a human
cell. The proviral deoxyribonucleic acid (DNA) is integrated into the host
genome in infected cells. Rarely, the provirus inserts near a protooncogene
(eg, int-1) and activates the protosoncogene. Activated protooncogene
results in cell transformation and in tumor formation. B, Sites of integration of MMTV retrovirus near the
protooncogene int-1. All sites determine int-1 activation.

Figure 6-3. Transfection assay. Deoxyribonucleic acid (DNA) from a tumor (eg,
bladder carcinoma) was used to transform a rodent immortalized cell line (NIH
3T3). After serial cycles, DNA from transformed cells was extracted and then
inserted into λ
vector, which was subsequently used to transform an appropriate Escherichia
coli strain. Using a specific probe (ALU), it was possible to
isolate and then characterize the involved human oncogene.

Figure 6-4. Effect of bcl-2 activity
on the control of the cell life. In the presence of BAX only, the cell goes to apoptosis; bcl-2
regulates the cycle of the cell by the interaction with BAX. When bcl-2
is overexpressed, the cell cycle is deregulated and the apoptosis is prevented,
eventually leading to tumor formation. This is an important cause for tumor
formation. PCD = programmed cell death or (apoptosis).
|
Table 6-1. Oncogenes |
|||||
|
|
|
|
|
|
|
|
Oncogene |
Chromosome |
Method of Identification
|
Neoplasm |
Mechanism of Activation
|
Protein Function |
|
Growth factors |
|
|
|
|
|
|
v-sis |
22q12.3–13.1 |
Sequence homology |
Glioma/fibrosarcoma |
Constitutive production
|
B-chain PDGF |
|
int2 |
11q13 |
Proviral insertion |
Mammary carcinoma |
Constitutive production
|
Member of FGF family |
|
KS3 |
11q13.3 |
DNA transfection |
Kaposi sarcoma |
Constitutive production
|
Member of FGF family |
|
HST |
11q13.3 |
DNA transfection |
Stomach carcinoma |
Constitutive production
|
Member of FGF family |
|
Growth factor receptors |
|
|
|
|
|
|
Tyrosine kinases: integral membrane
proteins |
|
|
|
|
|
|
EGFR |
7p1.1–1.3 |
DNA amplification |
Squamous cell carcinoma
|
Gene amplification/increased protein |
EGF receptor |
|
v-fms |
5q33–34 (FMS) |
Viral homolog |
Sarcoma |
Constitutive activation
|
CSF1 receptor |
|
v-kit |
4q11–21 (KIT) |
Viral homolog |
Sarcoma |
Constitutive activation
|
Stem-cell factor receptor |
|
v-ros |
6q22 (ROS) |
Viral homolog |
Sarcoma |
Constitutive activation
|
? |
|
MET |
7p31 |
DNA transfection |
MNNG-treated
human osteocarcinoma cell line |
DNA
rearrangement/ligand-independent constitutive activation (fusion proteins) |
HGF/SF receptor |
|
TRK |
1q32–41 |
DNA transfection |
Colon/thyroid carcinomas
|
DNA
rearrangement/ligand-independent constitutive activation (fusion proteins) |
NGF receptor |
|
NEU |
17q11.2–12 |
Point mutation/DNA amplification
|
Neuroblastoma/breast carcinoma
|
Gene amplification |
? |
|
RET |
10q11.2 |
DNA transfection |
Carcinomas
of thyroid; MEN2A, MEN2B |
DNA
rearrangement/point mutation (ligand-independent constitutive
activation/fusion proteins) |
GDNF/NTT/ART/PSP
receptor |
|
Receptors
lacking protein kinase activity |
|
|
|
|
|
|
mas |
6q24–27 |
DNA transfection |
Epidermoid carcinoma |
Rearrangement of 5?
noncoding region |
Angiotensin receptor |
|
Signal transducers |
|
|
|
|
|
|
Cytoplasmic tyrosine kinases |
|
|
|
|
|
|
SRC |
20p12–13 |
Viral homolog |
Colon carcinoma |
Constitutive activation
|
Protein tyrosine kinase |
|
v-yes |
18q21-3 (YES) |
Viral homolog |
Sarcoma |
Constitutive activation
|
Protein tyrosine kinase |
|
v-fgr |
1p36.1–36.2 (FGR) |
Viral homolog |
Sarcoma |
Constitutive activation
|
Protein tyrosine kinase |
|
v-fes |
15q25–26 (FES) |
Viral homolog |
Sarcoma |
Constitutive activation
|
Protein tyrosine kinase |
|
ABL |
9q34.1 |
Chromosome |
CML |
DNA
rearrangement translocation (constitutive activation/fusion proteins) |
Protein tyrosine kinase |
|
Membrane-associated G proteins |
|
|
|
|
|
|
H-RAS |
11p15.5 |
Viral homolog/ DNA transfection
|
Colon, lung, pancreas carcinmoas |
Point mutation |
GTPase |
|
RAS |
12p11.1–12.1 |
Viral homolog/ DNA transfection
|
AML, thyroid carcinoma, melanoma |
Point mutation |
GTPase |
|
N-RAS |
1p11–13 |
DNA transfection |
Carcinoma, melanoma |
Point mutation |
GTPase |
|
gsp |
20 |
DNA sequencing |
Adenomas of thyroid |
Point mutation |
Gs ? |
|
gip |
3 |
DNA sequencing |
Ovary, adrenal carcinoma
|
Point mutation |
Gi ? |
|
GTPase exchange factor (GEF) |
|
|
|
|
|
|
Dbl |
Xq27 |
DNA transfection |
Diffuse B-cell lymphoma
|
DNA rearrangement |
GEF for |
|
Vav |
19p13.2 |
DNA transfection |
Hematopoietic cells |
DNA rearrangement |
GEF for Ras? |
|
Serine/threonine kinases: cytoplasmic |
|
|
|
|
|
|
v-mos |
8q11 (MOS) |
Viral homolog |
Sarcoma |
Constitutive activation
|
Protein kinase (ser/thr) |
|
v-raf |
3p25 (RAF-1) |
Viral homolog |
Sarcoma |
Constitutive activation
|
Protein kinase (ser/thr) |
|
pim-1 |
6p21 (PIM-). |
Insertional mutagenesis
|
T-cell lymphoma |
Constitutive activation
|
Protein kinase (ser/thr) |
|
Cytoplasmic regulators |
|
|
|
|
|
|
v-crk |
17p13 (CRK) |
Viral homolog |
|
Constitutive
tyrosine phosphorilation of cellular substrates (eg, paxillin) |
SH-2/SH-3 adaptor |
|
Trancription Factors |
|
|
|
|
|
|
v-myc |
8q24.1 (MYC) |
Viral homolog |
Carcinoma, myelocytomatosis
|
Deregulated activity |
Transcription factor |
|
N-MYC |
2p24 |
DNA amplification |
Neuroblastoma; lung carcinoma
|
Deregulated activity |
Transcription factor |
|
L-MYC |
1p32 |
DNA amplification |
Carcinoma of lung |
Deregulated activity |
Transcription factor |
|
v-myb |
6q22–24 |
Viral homolog |
Myeloblastosis |
Deregulated activity |
Transcription factor |
|
v-fos |
14q21–22 |
Viral homolog |
Osteosarcoma |
Deregulated activity |
Transcription factor API |
|
v-jun |
p31–32 |
Viral homolog |
Sarcoma |
Deregulated activity |
Transcription factor API |
|
v-ski |
1q22–24 |
Viral homolog |
Carcinoma |
Deregulated activity |
Transcription factor |
|
v-rel |
2p12–14 |
Viral homolog |
Lymphatic leukemia |
Deregulated activity |
Mutant NFKB |
|
v-ets-1 |
11p23–q24 |
Viral homolog |
Erythroblastosis |
Deregulated activity |
Transcription factor |
|
v-ets-2 |
21q24.3 |
Viral homolog |
Erythroblastosis |
Deregulated activity |
Transcription factor |
|
v-erbA1 |
17p11–21 |
Viral homolog |
Erythroblastosis |
Deregulated activity |
T3 Transcription factor |
|
v-erbA2 |
3p22–24.1 |
Viral homolog |
Erythroblastosis |
Deregulated activity |
T3 Transcription factor |
|
Others |
|
|
|
|
|
|
BCL2 |
18q21.3 |
Chromosomal translocation
|
B-cell lymphomas |
Constitutive activity
|
Antiapoptotic protein |
|
MDM2 |
12q14 |
DNA amplification |
Sarcomas |
Gene amplification/increased protein |
Complexes with p53 |
AML =
acute myeloid leukemia; CML = chronic myelogenous leukemia; CSF = colony
stimulating factor; DNA = deoxyribonucleic acid; EGF = epidermal growth factor;
FGF = fibroblast growth factor; GTPase = guanosine triphosphatase; HGF =
hepatocyte growth factor; NGF = nerve growth factor; PDGF = platelet-derived
growth factor.

Figure 6-5. Schematic representation of the
main mechanisms of oncogene activation (from protooncogenes to oncogenes). The normal gene (protooncogene) is depicted with
its transcibed portion (rectangle). In the case of gene amplification, the
latter can be duplicated 100-fold, resulting in an excess of normal protein. A
similar situation can occur when following chromosome rearrangements such as
translocation, the transcription of the gene is now regulated by novel
regulatory sequences belonging to another gene. In the case of point mutation,
single aminoacid substitutions can alter the biochemical properties of the gene
product, causing, in the example, its constitutive enzymatic activation.
Chromosome rearrangements, such as translocation and inversion, can then
generate fusion transcripts resulting in chimeric oncogenic proteins.

Figure 6-6. C-myc translocations found
in Burkitt lymphoma. A, t(8;14)(q24;q32)
translocation involving the locus of immunoglobulin heavy-chain gene located at
14q32. B, t(8;14)(q24;q32) translocation where only 2 exons (Ex) of c-myc
are translocated under regulatory elements from the immunoglobulin heavy-chain
locus located at 14q32. C, t(8;22)(q24;q11) translocation involving the l
locus of immunoglobulin light-chain gene at 22q11. D, t(2;8)(p12;q24)
translocation involving the κ locus of immunoglobulin light-chain gene located at 2p12.

Figure 6-7. Gene fusion. The t(9;22)(q34;q11) translocation in chronic
myelogenous leukemia (CML) determines the fusion of the c-abl gene with
the bcr gene. Such a gene fusion encodes an oncogenic chimeric protein
of 210 kDa. Chr = chromosome.
|
Table 6-2. Oncogene Amplification in Human
Cancers |
|||
|
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|||
|
Tumor
Type |
Gene
Amplified |
Percentage |
|
|
|
|||
|
|
|||
|
Neuroblastoma |
MYCN |
20–25 |
|
|
Small-cell
lung cancer |
MYC |
15–20 |
|
|
Glioblastoma |
ERB
B-1 (EGFR) |
33–50 |
|
|
Breast
cancer |
MYC |
20 |
|
|
|
ERB
B-2 (EGFR2) |
~20 |
|
|
|
FGFR1 |
12 |
|
|
|
FGFR2 |
12 |
|
|
|
CCND1 (cyclin D1) |
15–20 |
|
|
Esophageal
cancer |
MYC |
38 |
|
|
|
CCND1 (cyclin D1) |
25 |
|
|
Gastric
cancer |
K-RAS |
10 |
|
|
|
CCNE (cyclin E) |
15 |
|
|
Hepatocellular
cancer |
CCND1 (cyclin D1) |
13 |
|
|
Sarcoma |
MDM2 |
10–30 |
|
|
|
CDK4 |
11 |
|
|
Cervical
cancer |
MYC |
25–50 |
|
|
Ovarian
cancer |
MYC |
20–30 |
|
|
|
ERB
B-2 (EGFR2) |
15–30 |
|
|
|
AKT2 |
12 |
|
|
Head
and neck cancer |
MYC |
7–10 |
|
|
|
ERB
B-1(EGFR) |
10 |
|
|
|
CCND1(cyclin D1) |
~50 |
|
|
Colorectal
cancer |
MYB |
15–20 |
|
|
|
H-RAS |
29 |
|
|
|
K-RAS |
22 |
|
|
Table
6-3. Neuroblastoma |
||
|
Benign
ganglioneuromas |
0/64(0%) |
100 |
|
Low
stages |
31/772 (4%) |
90 |
|
Stage
4-S |
15/190 (8%) |
80 |
|
Advanced
stages |
612/1.974 (31%) |
30 |
|
Total |
658/3000 (22%) |
50 |
MYCN copy numbers are correlated with stage and
survival in neuroblastoma.
|
|||
|
|
|
|
|
|
|
Rearrangements |
Disease |
Protein
Type |
|
|
|
|
|
|
|
|
|
|
|
c-ABL (9q34) |
t(9:22)
(q34:q11) |
CML and
acute leukemia |
Tyrosine kinase activated by BCR |
|
BCR (22q11) |
|
|
|
|
PBX-1(1q23) |
t(1:19)(q23:p13.3) |
Acute pre-B-cell leukemia |
Homeodomain |
|
E2A(19p13.3). |
HLH |
|
|
|
PML(15q21) |
t(15:17)
(q21:q11–22) |
Acute
myeloid leukemia |
Zinc
finger |
|
RAR(17q21) |
|
|
|
|
CAN(6p23) |
t(6:9)
(p23:q34) |
Acute
myeloid leukemia |
No
homology |
|
DEK(9q34). |
|
|
|
|
REL |
ins(2:12) (p13:p11.2–14) |
Non-Hodgkin
lymphoma |
NF(?)B family |
|
NRG |
|
|
No
homology |
|
Oncogenes
juxtaposed with IG loci |
|||
|
c-MYC |
t(8:14)
(q24:q32) |
Burkitt
lymphoma; BL-ALL |
HLH
domain |
|
|
t(2:8)
(p12:q24) |
|
|
|
|
t(8:22)
(q24:q11) |
|
|
|
BCL1
(PRADI?) |
t(11:14)
(q13:q32) |
B-cell chronic lymphocyte leukemia |
PRADI-GI
cyclin |
|
BCL-2 |
t(14:18)
(q32:21) |
Follicular
lymphoma |
Inner
mitochondrial membrane |
|
BCL-3 |
t(14:19)
(q32:q13.1) |
Chronic
B-cell leukemia |
CDC10
motif |
|
IL-3 |
t(5:14)
(q31:q32) |
Acute pre-B-cell leukemia |
Growth
factor |
|
Oncogenes juxtaposed with TCR loci |
|||
|
c-MYC |
t(8:14)
(q24:q11) |
Acute
T-cell leukemia |
HLH
domain |
|
LYLA |
t(7:19)
(q35:p13) |
Acute
T-cell leukemia |
HLH
domain |
|
TALA/SCL/TCL-5 |
t(1:14)
(q32:q11) |
Acute
T-cell leukemia |
HLH domain |
|
TAL-2 |
t(7:9)
(q35:q34) |
Acute
T-cell leukemia |
HLH
domain |
|
Rhombotin
1/Ttg-1 |
t(11:14)
(p15:q11) |
Acute
T-cell leukemia |
LIM
domain |
|
Rhombotin
2/Ttg-2 |
t(11:14)
(p13:q11) |
Acute
T-cell leukemia |
LIM
domain |
|
|
t(7:11)
(q35:p13) |
|
|
|
HOX 11 |
t(10:14)
(q24:q11) |
Acute
T-cell leukemia |
Homeodomain |
|
|
t(7:10)
(q35:q24) |
|
|
|
TAN-1 |
t(7:9)
(q34:q34.3) |
Acute
T-cell leukemia |
Notch
homologue |
|
TCL-1 |
t(7q35-14q32.1) |
B-cell chronic lymphocitic leukemia |
|
|
|
or inv |
|
|
|
|
t(14q11-14q32.1) |
|
|
|
|
or inv |
|
|
|
Solid
Tumors |
|
|
|
|
Gene fusions in sarcomas |
|
||
|
FLI1,EWS |
t(11:22)
(q24:q12) |
Ewing sarcoma |
Ets
transcription factor family |
|
ERG,EWS |
t(21:22)
(q22:q12) |
Ewing sarcoma |
Ets
transcription factor family |
|
ATV1,EWS |
t(7:21)
(q22:q12) |
Ewing sarcoma |
Ets
transcription factor family |
|
ATF1,EWS |
t(12:22)
(q13:q12) |
Soft-tissue clear cell sarcoma |
Transcription
factor |
|
CHN,EWS |
t(9:22)
(q22 31:q12) |
Myxoid chondrosarcoma |
Steroid
receptor family |
|
WT1,EWS |
t(11:22)
(p13:q12) |
Desmoplastic small round cell tumor |
Wilms
tumor gene |
|
SSX1,SSX2,SYT |
t(X:18) (p11.2:q11.2) |
Synovial
sarcoma HLH domain |
|
|
PAX3,FKHR |
t(2:13)
(q37:q14) |
Alveolar
rhabdomysarcoma |
Homeobox
homologue |
|
PAX7,FKHR |
t(1:13)
(q36:q14) |
Rhabdomyosarcoma |
Homeobox
homologue |
|
CHOP,TLS |
t(12:16)
(q13:p11) |
Myxoid liposarcoma |
Transcription
factor |
|
var,HMG1-C |
t(var:12) (var:q13–15) |
Lipomas |
HMG
DNA-binding protein |
|
HMG1-C?
|
t(12:14)
(q13–15) |
Leiomyomas |
HMG
DNA-binding protein |
|
Gene
fusions in thyroid carcinomas |
|||
|
RET/ptc1 |
inv(10) (q11.2:q2.1) |
Papillary
thyroid carcinomas |
Tyrosine kinase actived by H4 |
|
RET/ptc2 |
t(10:17)
(q11.2:q23) |
Papillary
thyroid carcinomas |
Tyrosine kinase actived by RIa(PKA) |
|
RET/ptc3 |
inv(10) (q11.2) |
Papillary
thyroid carcinomas |
Tyrosine kinase actived by ELE1 |
|
TRK |
inv(1) (q31:q22–23) |
Papillary
thyroid carcinomas |
Tyrosine kinase actived by TPM3 |
|
TRK-T1(T2) |
inv(1) (q31:q25) |
Papillary
thyroid carcinomas |
Tyrosine kinase actived by TPR |
|
TRK -T3 |
t(1q31:3) |
Papillary
thyroid carcinomas |
Tyrosine kinase actived by TFG |
|
Haematopoietic
and solid tumors |
|||
|
Oncogenes
juxtaposed with other loci |
|||
|
PTH deregulates PRAD1 |
inv(11)(p15:q13) |
Parathyroid
adenoma |
PRADI-GI
cyclin |
|
BTG1
deregulates MYC |
t(8:12)(q24:q22) |
B-cell
chronic lymphocytic |
MYC-HLH
domain |
HLH = helix loop helix
structural domain; HMG = high mobility group; H4; ELE1; IG = immunoglobulin;
TPR and TFG = partially uncharacterized genes with a dimerizing coiled-coil
domain; RIa = regulatory subunit of PKA enzyme; TCR =
T-cell receptor; TPM3 = isoform of nonmuscle tropomyosin.

Figure 6-8. A model of exposure to a mutagen and to a tumor
promoter. Cancer develops
exclusively when the exposure to promoter follows the exposure to carcinogen
(mutagen; eg, 7,12-dimethyl-benzanthracene [DMBA]) and
only when the intensity of the exposure to promoter is higher than a threshold.

Figure 6-9. Colorectal cancer development. Colorectal cancer results from a series of
pathologic changes that transform normal colonic epithelium into invasive
carcinoma. Specific genetic events, shown by vertical arrows, accompany this
multistep process.

Figure 6-10. Mode of action of STI571. The effect of ATP binding on the oncoprotein
BCR-ABL (left): the fusion protein binds the molecule of ATP in the kinase
pocket. Afterwards, it can phosphorylate a substrate, that
can interact with the downstream effector molecules. When STI571 is present
(right), the oncoprotein binds STI571 in the kinase pocket (competing with
ATP); therefore the substrate cannot be phosphorylated.

Figure 6-11. Paracrine and autocrine stimulation. A, A growth factor produced by the cell on the right stimulates another
cell carrying the appropriate receptor (left) on cell membrane. This process is
named paracrine stimulation. B, A growth factor is produced by the same cell expressing the
corresponding receptor. This process is designated autocrine stimulation.

Figure 6-12. Representative examples of tyrosine kinase receptor
families. EGF = epidermal growth
factor; FGF = fibroblast growth factor; Ig= immunoglobulin; IGF1 = insulinlike
growth factor; PDGF = platelet-derived growth factor; VEGF = vascular
endothelial growth factor.